CS MEDICA A/S Interim Report Q3 (April - June 2025)
CS MEDICA reports Q3 results for April–June 2025, marked by strengthened cost control, solid regulatory progress, and a significantly improved operating result despite lower revenue due to postponed deliveries.
Financial Performance
-
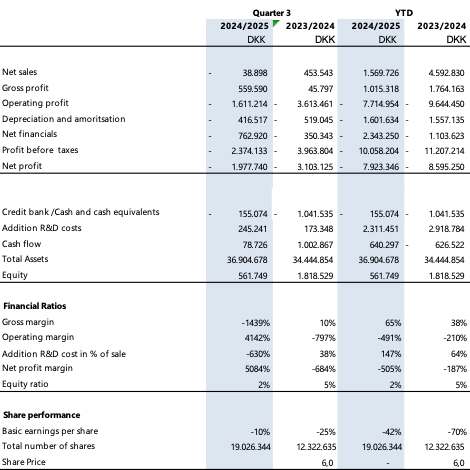
Operating result (EBIT): DKK –1.6 million, an improvement from DKK –3.6 million in Q3 last year.
-
Order intake: DKK 2.2 million, supported by two new EU customers, a major own-label order, and renewed demand.
-
Pipeline: approx. DKK 20 million, with DKK 6 million in ongoing execution.
Regulatory & Market Progress
The Group advanced its MDR transition with BSI, including surveillance audit completion and ongoing technical assessments. Nasal Protect Gel and Psoriasis Gel were approved in Thailand, while reviews progressed in Jordan and China’s Hainan Pilot Zone. The Psoriasis Gel patent was granted in Australia.
Outlook
Management expects higher revenue in Q4, continued operational stabilisation, and strengthened regulatory momentum.